The β bulge () is a small piece of nonrepetitive structure that can occur by itself in the coil regions, but which most often occurs, and is most easily visualized, as an irregularity in antiparallel β structure.
A β bulge is defined as a region between two consecutive β-type hydrogen bonds that includes two residues on one strand opposite a single residue on the other strand. Figure 40 shows a β bulge from trypsin. The two residues on the bulged strand are called positions 1 and 2, and the one on the opposite strand position X. Sometimes the hydrogen bond to the CO of position X is forked, coming from the NH groups of both positions 1 and 2; the bulge in Fig. 40 shows this feature.
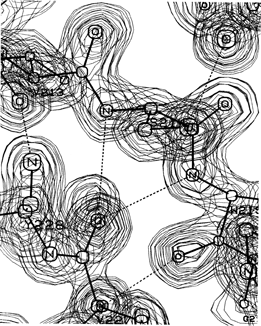
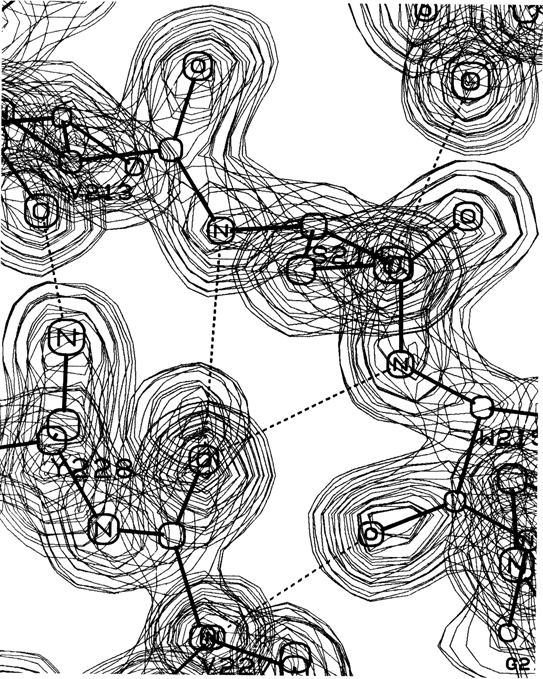
FIG. 40. A classic β bulge: the model and electron density from refined trypsin residues Ser-214, Trp-215, and Val-227. Courtesy of Chambers and Stroud.
Only about 5% of the β bulges are between parallel strands, and most of the antiparallel ones are between a closely spaced (see Section II,B) rather than a widely spaced pair of hydrogen bonds. The additional backbone length of the extra residue on the longer side is accommodated partly by bulging that strand to the right and toward you as seen in Fig. 40 and partly by putting a slight bend in the β sheet.
Like tight turns, bulges affect the directionality of the polypeptide chain, but in a much less drastic manner. β bulges are not as common as tight turns, but over a hundred examples are known (see , for a listing of 91). [A survey by Chan et al. (1993) covers much larger numbers.]
β bulges can be classified into several different types, which are illustrated schematically in Fig. 41. By far the commonest is the "classic" β bulge, which occurs between a narrow pair of hydrogen bonds on antiparallel strands and has the side chains of positions 1, 2, and X all on the same side of the β sheet (see Fig. 41a). Residue 1 is in approximately α-helical conformation (averaging φ1 = -100°, ψ1 = -45°) and residues 2 and X in approximately normal β conformation (averaging φ2 = -140°, ψ2 = 160°, and φx = -100°, ψx = 130°). Figure 42 is a stereo drawing of five examples of classic β bulges superimposed on one another. Note that the carbonyl oxygens on either side of residue 1 both point in about the same direction, as is typical of α-helical conformation, while the carbonyls surrounding residues 2 and X point opposite each other, as in β structure. Figure 42 also shows that a classic bulge locally accentuates the normal right-handed twist (see Section II,B) of the β strands.
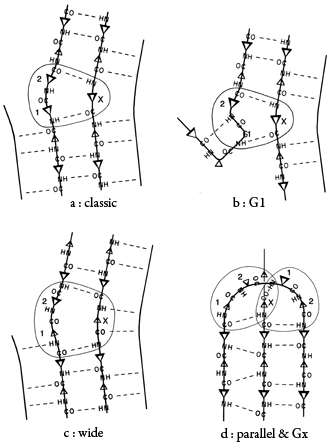
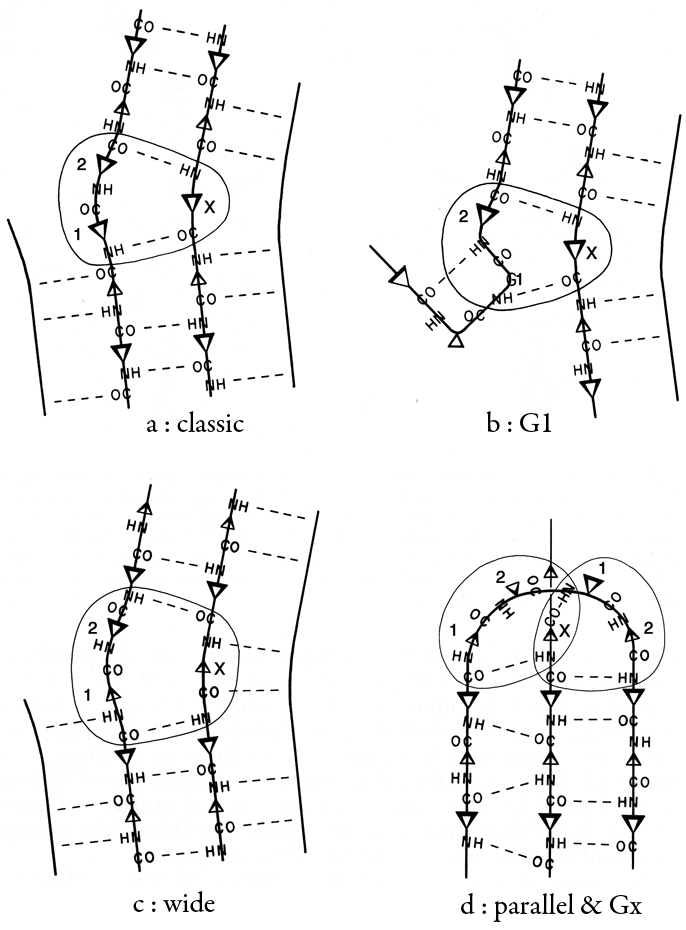
FIG. 41. Diagrammatic illustrations of various types of β bulges (circled): (a) a classic β bulge; (b) a G1 β bulge, with the associated type II tight turn; (c) a wide β bulge; (d) a + 2x connection which forms a parallel β bulge on the left side and a Gx bulge on the right. Positions 1, 2, and X of the bulge are labeled. Small triangles represent side chains that are below the sheet, and larger triangles those that are above it.
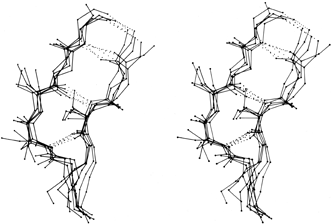
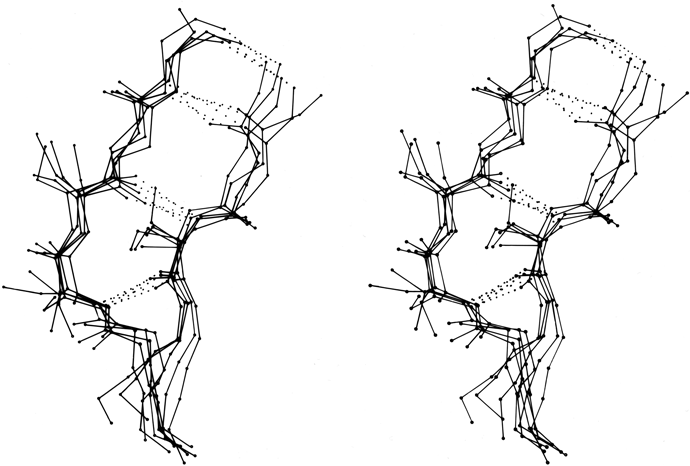
FIG. 42. Five superimposed examples of classic β bulges, in stereo: chymotrypsin Phe-41, Cys-42 opposite Leu-33; chymotrypsin Ala-86, Lys-87 opposite Lys-107; concanavalin A Leu-107, Ser-108 opposite Ala-196; carbonic anhydrase C Ile-90, Gln-91 opposite Val-120; and staphylococcal nuclease Ile-15, Lys-16 opposite Lys-24. Here and in Fig. 43 side chains are shown (out to Cγ) only for the three positions within the bulge. At the very bottom of this figure only the backbone is shown, where the two strands overlap in this projection.
The next most common type is the G1 bulge, illustrated schematically in Fig. 41b. It also lies between a narrow pair of hydrogen bonds, and position 1 is almost invariably a glycine because of its backbone conformation: φ1 ≅ 85°, ψ1 ≅ 0° and φ2 ≅ -90°, ψ2 ≅ 150°. More than half of the G1 bulges are found within an interlocking structure in which the glycine in position 1 of the G1 bulge is also the required glycine in position 3 of a type II tight turn (see preceding section). The plane of the tight turn and its hydrogen bond is almost perpendicular to the plane of the G1 bulge. This combined structure has a consistent handedness which is dictated by the requirements of the three hydrogen bonds. A set of G1 bulges with their associated tight turns are shown superimposed in stereo in Fig. 43. For G1 bulges, the side chain of the glycine (if it had one) would be on the opposite side of the β sheet from those in positions 2 and X. In contrast to classic bulges, G1 bulges seldom have continued β structure on the bottom end (as seen in Fig. 41b or 43); when there is an associated tight turn the bulged strand enters at a very sharp angle, and in many of the remaining cases there is a short connection between the two strands of the bulge (so that position 1 equals either χ + 3 or χ + 4). [Such "β-bulge loops" are treated separately by Milner-White (1987).]
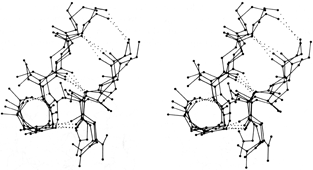
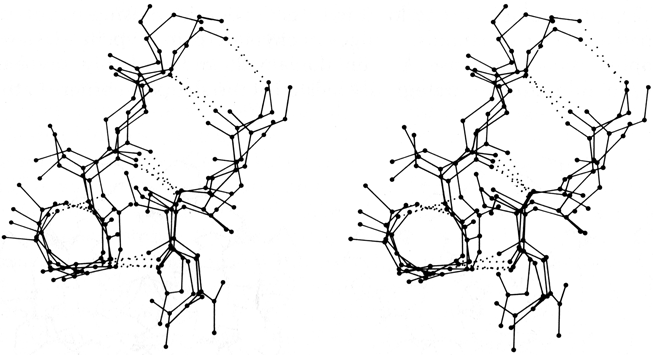
FIG. 43. Four superimposed examples of G1 β bulges with associated type II tight turns: trypsin Gly-133, Thr-134 opposite Ile-162; elastase Gly-204, Gly-205 opposite Thr-221; Bence-Jones REI VL Gly-16, Asp-17 opposite Leu-78; and cytochrome c Gly-37, Arg-38 opposite Trp-59. The tight turn is the small hydrogen-bonded loop at the lower left; its plane is approximately perpendicular to the plane of the bulge. The α-carbon in the lower right corner of the loop is the required glycine in position 3 of the turn and position 1 of the bulge.
"Wide type" β bulges are those that occur between a widely spaced pair of hydrogen bonds on antiparallel strands, as shown schematically in Fig. 41c. They apparently are much less constrained than narrow bulges, since they occur with a great variety of backbone conformations; however, they do not occur as often. Even more unusual are the Gx bulges (so named because they often have a glycine in position X) with a hydrogen-bonding pattern similar to that shown in Fig. 41d, and the parallel bulges, which can take several forms, one of which is also illustrated in Fig. 41d.
Bulges have the general property of causing the normal β sheet alternation of side chain direction to be out of register on the two ends of one of the bulge strands. The bulge can be thought of as turning over one-half of the out-of-register strand; the classic bulge accomplishes this by changing the ψ angle of the residue in position 1 by about 180°, which moves it from the β conformational region to the α region. Once half of the strand has flipped over, it must shift sideways by one residue along the other strand in order to hydrogen bond; that shift produces the bulge of two residues opposite one.
Bulges, as well as tight turns, are very often found at active sites, probably because they have a strictly local but specific and controlled effect on side chain direction. Another possible function for β bulges would be as a mechanism for accommodating a single-residue insertion or deletion mutation without totally disrupting the β sheet. There seem to be several such cases in the immunoglobulins, the clearest of which is a one-residue insertion in the CH1 domain of Fab'NEW relative to the sequence of McPC603 CH1: the NEW structure has a bulge in the middle of a long pair of β strands, while in McPC603 those strands form regular β structure all the way along.
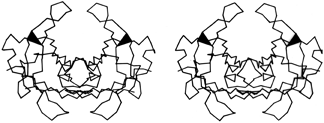
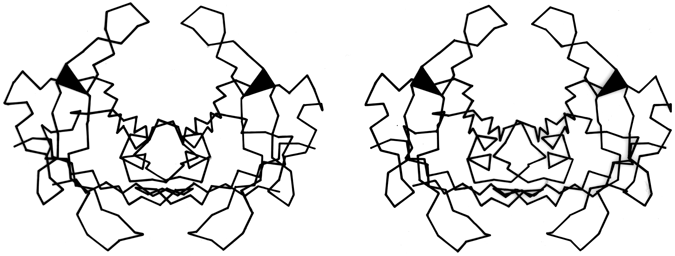
FIG. 44. Stereo view of the prealbumin dimer. The black triangles are β bulges which help to turn outward the β ribbons that form the loops proposed as a possible site for binding double-helical DNA. [Those loops turn out not, in fact, to bind DNA.]
The other general property of β bulges is that they alter the direction of the backbone strands forming them; classic bulges also accentuate the right-handed twist of the strands. This means that bulges are often useful for shaping large features of β sheet and/or extended hairpin loops. In antiparallel β barrels, for instance (see Section II,B), an extremely strong local twist is needed for closing barrels as small as five or six strands. Bulges in chymotrypsin, trypsin, elastase, staphylococcal nuclease, papain domain 2, and probably soybean trypsin inhibitor are strategically located at the sharpest corners in the β strands. Extended two-strand β ribbons are often used for forming external interaction sites on a protein. Such a ribbon would normally extend from the end of a β sheet or β barrel, but if the interaction site needs to be at one side of the sheet or barrel, then a β bulge at the point of departure can be used to direct the β ribbon out more nearly at right angles. For example, in the immunoglobulins the 47,48;35 bulge in the VL domain and the 48,49;36 bulge in the VH domain send out a pair of β ribbons that help complete closure around the VL—VH interdomain contact. In prealbumin the Phe-44,Ala-45;Val-32 bulge helps to turn out the extended VL ribbon near the 2-fold axis of the dimer that forms the DNA-binding site proposed in Blake and Oatley () (see Fig. 44).