Disulfide bridges are, of course, true covalent bonds (between the sulfurs of two cysteine side chains) and are thus considered part of the primary structure of a protein by most definitions. Experimentally they also belong there, since they can be determined as part of, or an extension of, an amino acid sequence determination. [This was true when sequencing was done on proteins, but of course DNA sequencing does not determine SS connectivity.] However, proteins normally can fold up correctly without or before disulfide formation, and those SS links appear to influence the structure more in the manner of secondary-structural elements, by providing local specificity and stabilization. Therefore, it seems appropriate to consider them here along with the other basic elements making up three-dimensional protein structure.
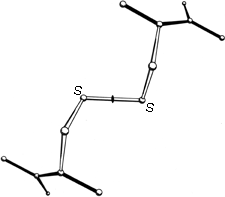
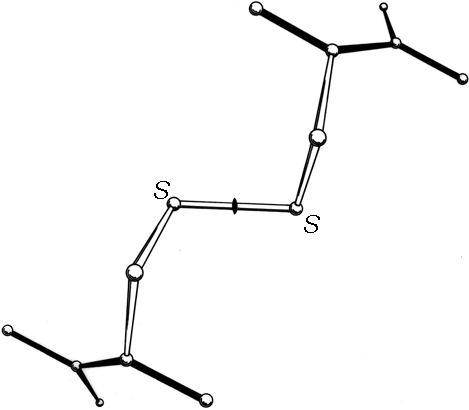
FIG. 45. The structure of L-cystine dihydrobromide, seen down the crystallographic 2-fold axis. The disulfide is in a left-handed spiral conformation.
A modest amount of accurate conformational information is available from small-molecule X-ray structures of various forms of cystine. χ1 angles are close to +60°, and all three dihedral angles internal to the disulfide are close to + or -90°. Two mirror-image conformations are observed; in N,N'-diglycyl-L-cystine dihydrate () and in L-cystine dihydrobromide () or hydrochloride () all three internal dihedral angles are approximately -90°, forming a left-handed spiral, while in hexagonal L-cystine (, ) they are all approximately +90°, forming a right-handed spiral. Figure 45 shows the left-handed disulfide of L-cystine dihydrobromide, viewed down the 2-fold axis perpendicular to the S—S bond.
The dihedral angles of disulfides in proteins are very difficult to determine with any accuracy except in refined high-resolution structures. In the first few protein structures to show disulfides at 2 Å resolution, attention was paid mostly to the dihedral angle around the S—S bond (χ1), since it is the most characteristically interesting parameter for cystine, is one of the easier ones to measure, and presumably is correlated with the handedness of the large optical rotation associated with the presence of disulfide bridges. As expected, the χ3 angles were in the range around 90-100° and were found in both left-handed and right-handed forms (; ).
[By now of course, a great many more SS-containing structures have been determined, and better statistics are available (see below). Oddly, however, SS conformation is the least well determined of any side chain because 1) they are relatively rare, 2) series-termination ripples around the S atoms can distort the electron density, and 3) the fact that they are connected on both ends makes any misfittings difficult to correct.]
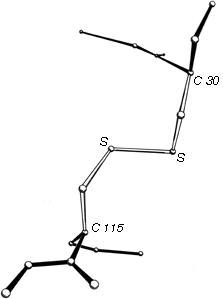
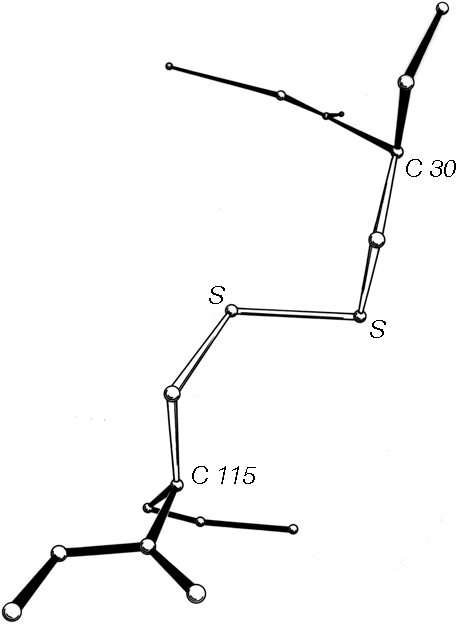
FIG. 46. A left-handed spiral disulfide from hen egg white lysozyme, viewed from a direction similar to Fig. 45.
Now that about 70 different disulfides have been seen in proteins and more than 20 of those have been refined at high resolution, it is possible to examine disulfide conformation in more detail, as it occurs in proteins. Many examples resemble the left-handed small-molecule structures extremely closely; Fig. 46 shows the Cys-30-Cys-115 disulfide from egg white lysozyme. The χ2, χ3, and χ2' dihedral angles and the Cα—Cα' distance can be almost exactly superimposed on Fig. 45; the only major difference is in χ1. All of the small-molecule structures have χ1 close to 60°. Figure 47 shows the χ1 values for half-cystines found in proteins. The preferred value is -60° (which puts Sγ trans- to the peptide carbonyl), while 60° is quite rare since it produces unfavorable bumps between Sγ and the main chain except with a few specific combinations of χ2 value and backbone conformation.
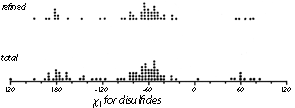
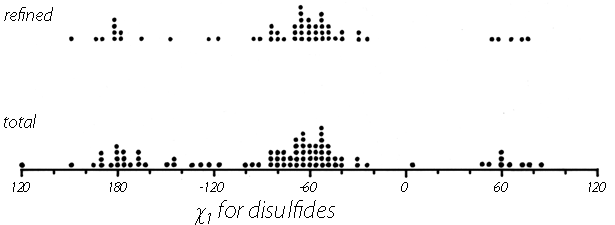
FIG. 47. The χ1 angles observed for disulfides in protein structures. The examples from refined, high-resolution structures are shown separately at the top.
[Add Fig. for current data ??]
If χ3 is kept between 90° and 100° and χ2 and χ2' are varied, the distance between α-carbons across the disulfide can range all the way from about 4 to 9Å. That corresponds to the extreme range observed for all disulfides. However, the effective range for Cα—Cα' distance is really a good deal narrower than that, since 85% of all the disulfides and 95% of the refined ones fall between 4.4 and 6.8Å.
Now let us examine the relationships between handedness of χ3, Cα—Cα distance, and χ2 values. Among the disulfides for which coordinates were available at 2 Å resolution or better (; ; ; ; ; ; ; ; ; Brookhaven Data Bank, (1980) ), there are equal numbers with right-handed and left-handed χ3. The average Cα—Cα' distance across the left-handed ones is 6.1 Å, exactly what was seen in the small-molecule structures, but for the righthanded ones the average Cα—Cα' distance is 5.2 Å. [The equal numbers and the length difference, still holds true.] Clearly the two sets of disulfides as they occur in proteins cannot simply be mirror images of one another.
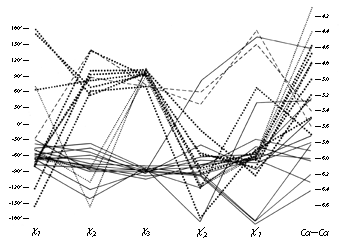
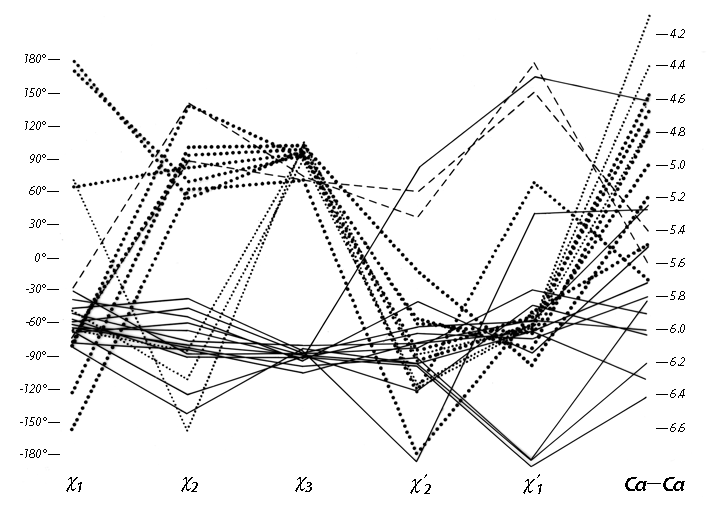
FIG. 48. Plot of all five dihedral angles and Cα—Cα distance for the disulfides from refined, high-resolution (2 Å or better) protein structures. The left-handed disulfides (almost all of which are left-handed spirals) are shown with solid lines, the - + + (right-handed hook) disulfides with large dots, the - + - (short right-handed hook) disulfides with small dots, and the + + + (right-handed spiral) disulfides with dashed lines. The long immunoglobulin disulfides are not included here.
Figure 48 plots all five side chain dihedral angles (χ1, χ2, χ3, χ2', and χ1') and the Cα—Cα' distances for the high-resolution disulfides. The overall conformations fall into just two major and two minor categories (plus perhaps one more to be discussed below). Essentially all of the left-handed ones (solid lines in Fig. 48) have approximately χ1 = -60°, χ2 = -90°, χ3 = -90°, χ2' = -90°, χ1' = -60°. This can be called the left-handed spiral conformation, and is the same as that seen in Fig. 46. An example from ribonuclease S is shown in Fig. 49, looking down the spiral. A majority of the right-handed disulfides have a conformation of approximately χ1 = -60°, χ2 = +120°, χ3 = +90°, χ2' = -50°, χ1' = -60° (heavy dots in Fig. 48) and a Cα separation averaging only 5 Å. This can be called the right-handed hook conformation; a typical example is shown in Fig. 50. Two cases have - + - dihedral angles and a Cα—Cα distance of 4-4.5 Å; these could be called short right-handed hooks. [Now that the database is larger, it can be seen that these short - + - disulfides join residues opposite one another on adjacent antiparallel β strands. They have been christened "staples" by Sternberg. Although connecting β strands is a very useful function the SS staples are not very common, reflecting the fact that their conformation is actually somewhat strained.] Then there are two cases of right-handed spirals (dashed lines in Fig. 48) which tend to have one χ1 angle of 180° and are still significantly shorter than the left-handed spirals. (ref ??) [?? is this still true?]
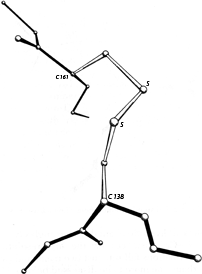
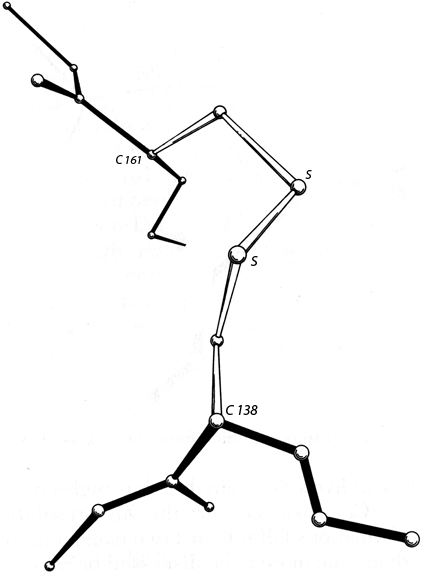
FIG. 50. A right-handed hook disulfide from carboxypeptidase A.
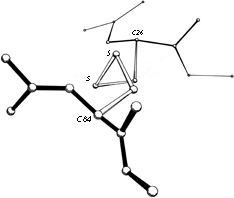
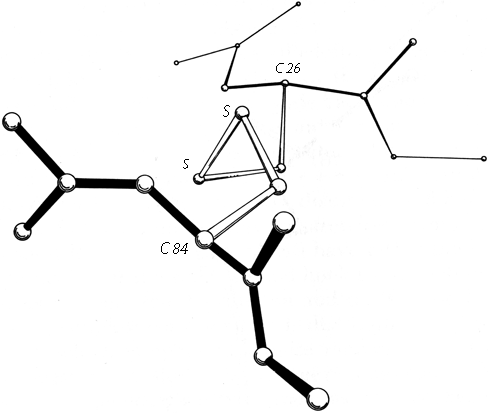
FIG. 49. A left-handed spiral disulfide from ribonuclease S, viewed end-on.
The only cases that were omitted from Fig. 48 are the disulfides that span the β barrels in immunoglobulins (; ). They are unusually long, with Cα separations of 6.6 to 7.4 Å, which is achieved by having both χ2 and χ2' close to 180°. These long disulfides are trans-gauche-trans (180°, +or-90°, 180°) in χ2, χ3, χ2', while both the spiral and the hook conformations described above are gauche-gauche-gauche (+or-90°, +or-90°, +or-90°). The χ1 angles are generally either 180 or 60° (in contrast to the usually preferred -60°) and the χ3s show no preference for left-handed vs right-handed. Indeed, in the Bence-Jones VL dimer REI () the disulfides were found to be a disordered mixture of the right-handed and left-handed forms. Asymmetrical preferences for the handedness of χ3 presumably must involve either direct or indirect interactions with the backbone; with χ2 near 180° such interactions are minimized. However, one must also presume that these unusually long disulfides are at least slightly strained and that χ2 angles in the 60-120° range are more favorable as a general rule. It also may well be true that χ3 is even more strongly constrained to +90° than is χ2, but the angle distributions seen in Fig. 48 should not be taken as proof that that is so, because preferred values for χ3 and not for χ2 have been built into almost all model-building and refinement routines. Molecular orbital calculations for χ3 by Pullman and Pullman () give an energy minimum at 100°, but do not rise by more than 0.5 kcal/mol from about 70 to 140°. They did not report calculations for χ2. [?? Note Hermans or other calc's?]
The distinctive differences between the left- and right-handed disulfide conformations have little to do with the χ3 angle itself. The bumps of the sulfurs with the polypeptide backbone are produced by a combination of χ1 and χ2; since it is unfavorable to have χ2 in the range of +60 to +100° when χ1 has its preferred value near -60°, the right-handed disulfides cannot adopt a +++++ spiral in proteins.
In summary, we can expect that most disulfides will have Cα separations of less than 6.5 Å unless they are stretched across a β barrel or perhaps a short loop. The majority will have either the left-handed spiral conformation or the right-handed hook conformation. [?? Summary for recent data?]
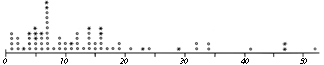
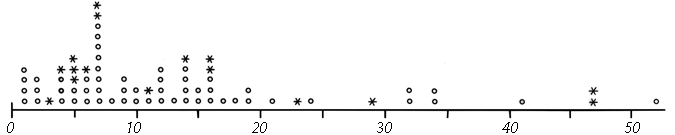
FIG. 51. Number of residues between sequence-neighbor half-cystines. Pairs which are in the same disulfide are shown by asterisks and those which are not by circles. [?? Update Fig.? Discussion of C(x)nC prefs, esp. disfavored c5c ??]
Now let us examine the distribution and position of disulfides in proteins. The simplest consideration is distribution in the sequence (see Fig. 51), which is apparently quite random, except that there must be at least two residues in between connected half-cystines. [Rare cases are now known with tighter spacing, which is thus quite unfavorable but not impossible.] Examples: ??] Even rather conspicuous patterns such as two consecutive half-cystines in separate disulfides turn out, when the distribution is plotted for the solved structures (Fig. 51), to occur at only about the random expected frequency. The sequence distribution of half-cystines is influenced by the statistics of close contacts in the three-dimensional structures, but apparently there are no strong preferences of the cystines that could influence the three-dimensional structure.
Disulfide topology may be considered in terms of the possible patterns of cross-bridges on a floppy string. The cases that occur appear to be a random selection among the possible alternatives, showing no evident preferences for or against any particular features (such as whether nearest-neighbor half-cystines are connected or whether the total connectivity can be drawn in two dimensions). This situation is a very marked contrast to what we found for β sheet topology, where there are a number of quite strong topological preferences. However, the topological and sequence randomness of disulfides is what one would expect if their major role is to stabilize close contacts in the final structure but they have no influence on the early stages of the protein-folding process.
We can also examine what types of backbone conformation are found at the ends of disulfides, and here we can see some preferences again. Well over half of the backbone strands are in extended conformation, although a relatively small fraction are actually part of a β sheet. It is not possible for a disulfide to join neighboring strands in a β sheet [(This has turned out not to be true: SS "staples" do so (see above).)] : any but the closest residues on adjacent strands are too far apart, and a closest pair of residues is slightly too close together. Also, for a close pair on β sheet the Cα-Cβ bonds of the two are approximately parallel, while they need to be approximately perpendicular for a right-handed hook and antiparallel for either right- or left-handed, spirals. Occasionally disulfides join next-nearest-neighbor β strands with only some disruption of the intervening hydrogen-bonding pattern (e.g., Cys-40-Cys-95 in ribonuclease S). An even more common relationship to β sheet is a disulfide joining the continuation of two nearest-neighbor strands after they have separated from the β sheet and can attain a more suitable separation and angle. Typically the disulfide is one or two residues out from the last hydrogen bond on one strand and three or four residues out on the other strand; the β strands are almost always antiparallel rather than parallel. This sort of arrangement is somewhat reminiscent of a β bulge (see Section II,D), and for the case of Cys-65-Cys-72 in ribonuclease S the disulfide actually spans one end of a wide type β bulge. [Another common arrangement in antiparallel β structure has two SS in contact, coming from residues directly opposite on the adjacent strands of a β-hairpin, one SS in a right-hand spiral and one in a left-hand spiral (Richardson Protein Tourist). This has been aptly named the SS β cross (Sternberg).]
α-Helix is also quite common as the backbone conformation flanking a disulfide, but there is seldom well-formed helix on both ends of a given disulfide. If one end comes from an α-helix, the other end will usually be an extended chain, or one or more tight turns, or irregular structure past the end of a helix. Presumably the constraints on favorable separation and angle of Cα—Cβ bonds in disulfides are difficult to satisfy with residues in any of the normal helix packing arrangements (see Section II,A). There are no disulfides at all in any of the helix-bundle structures (see Section III,B). Disulfides do connect a pair of adjacent helices, however, in phospholipase A2 and in crambin.
In cases in which backbone direction is readily definable (primarily for extended or helical chains), the two chains joined by a disulfide almost always cross at steep angles to one another (60 to 90°).
The third most frequent backbone conformation at disulfide ends is a tight turn. Sometimes it is a succession of turns, or bit of 310-helix. Turns seem to be somewhat favored at the hook end of a right-handed hook disulfide.


FIG. 52. A schematic backbone drawing of insulin, a small structure which is dependent on its disulfides for stability.
There is a correlation between the backbone conformations which commonly flank disulfides and the frequency with which disulfides occur in the different types of overall protein structure (see Section III,A for explanation of structure types), although it is unclear which preference is the cause and which the effect. There are very few disulfides in the antiparallel helical bundle proteins and [almost] none in proteins based on pure parallel β sheet (except for active-site disulfides such as in glutathione reductase). Antiparallel β sheet, mixed β sheet, and the miscellaneous α proteins have a half-cystine content of 0-5%. Small proteins with low secondary-structure content often have up to 15-20% half-cystine. Figure 52 shows the structure of insulin, one of the small proteins in which disulfides appear to play a major role in the organization and stability of the overall structure.