The next major grouping consists of domains that are organized around an antiparallel β sheet. They are as numerous as the parallel α/β structures, and their topology and classification have been discussed before (see ; ,; ). This category is the most varied in terms of size and organizational patterns. Figures 79 through 84 show backbone schematics for the antiparallel β domains, grouped into subcategories.
Most of the antiparallel β domains have their β sheets wrapped around into a cylinder, or barrel, shape. None of the antiparallel barrels has as symmetrical or as continuously hydrogen-bonded a cylindrical sheet as the singly wound parallel β barrels of triosephosphate isomerase and pyruvate kinase d1; however, antiparallel barrels are very much more common. Because of gaps in the hydrogen bonding, some of these structures have been described as two β sheets facing each other (e.g., ; ; ). Our reasons for treating them all as barrels are that the gap positions are sometimes different in domains that are probably related, and that the barrel description yields very much simpler and more unified topologies.
Barrels seem to prefer pure parallel or antiparallel β structure even more strongly than does β sheet in general. All the known singly wound barrels are pure parallel. An antiparallel barrel with an odd number of strands is constrained to have one parallel interaction, but no other parallel strand pairs occurs within antiparallel barrels except in the acid proteases. [ Of course, there are now more absolute numbers of such exceptions, but they are still quite rare. ] Also, even-stranded barrels are much more common than odd-stranded ones.
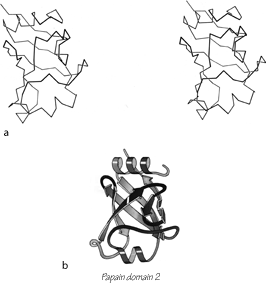
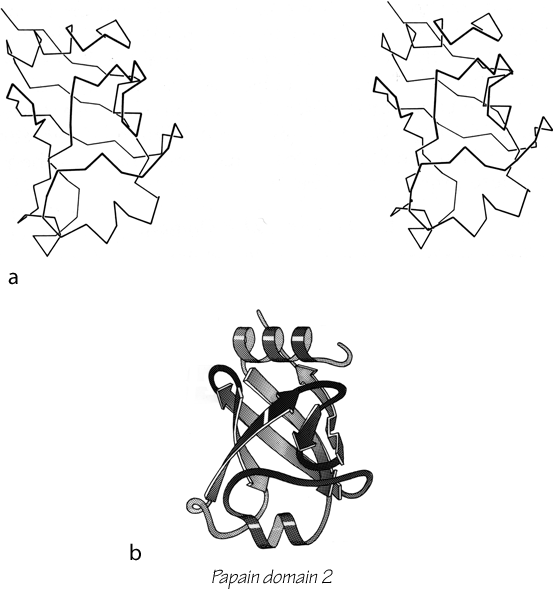
FIG. 95. Papain domain 2 as an example of an up-and-down antiparallel β barrel. (a) α-Carbon stereo, viewed from one side of the barrel; (b) backbone schematic, viewed as in a.
The first type of antiparallel β barrel, in analogy with the first type of helix bundle, has simple up and down +1, +1, +1 connections all around. Although it is relatively unusual for a barrel to be composed entirely of up-and-down strands, many of the larger barrels and sheets have four- to six-stranded sections of simple up-and-down topology embedded within them. There are three examples of pure up-and-down β barrels: soybean trypsin inhibitor, papain d1, and catalase d1. [ Two interesting later examples are retinol-binding protein (1KT7) and the large, membrane-spanning, 16-strand or more up-and-down barrel of porins (e.g. 1HXX). ] Figure 95 shows a stereo and a schematic drawing of papain d1. Soybean trypsin inhibitor has long excursions at the ends of three of the β strand pairs, forming separate, twisted β ribbons; there is a strong internal 3-fold symmetry which includes these ribbons as well as the strand pairs in the barrel (). Catalase d1 is an eight-stranded up-and-down barrel with less extreme loop excursions. Rubredoxin could be considered as a very irregular and incomplete up-and-down barrel in which β-type hydrogen bonds are formed between only about half of the strand pairs (see Fig. 76). It is very small and compact, and is presumably stabilized partly by the network of Cys ligands to the iron; therefore we have placed it in the small metal-rich category.
Soybean trypsin inhibitor, papain d1, and rubredoxin have identical topologies: six strands of +1, +1, +1, . . . proceeding to the left around the barrel if the chain termini are at the bottom. However handedness is not nearly as meaningful a property for up-and-down topologies as it is for Greek keys, since up-and-down handedness can change on addition or defection of a single strand.
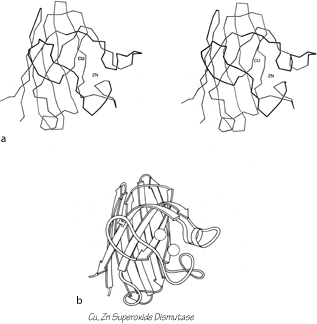
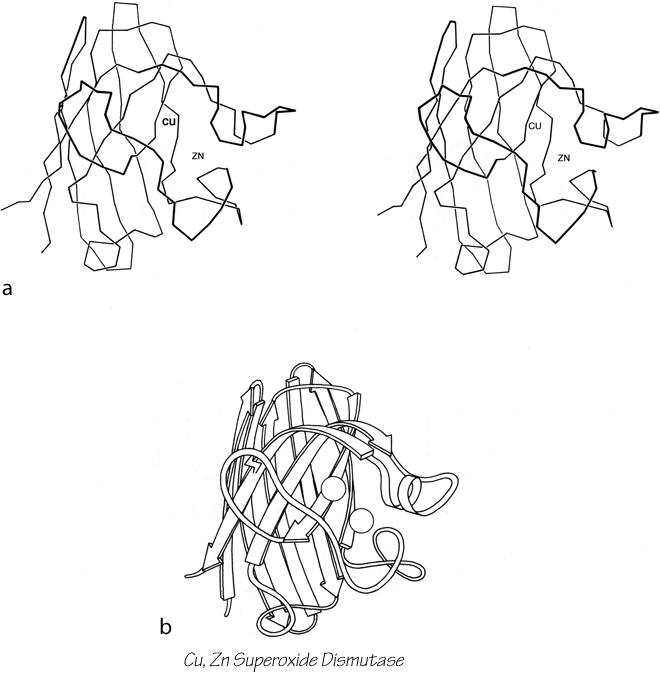
FIG. 96. Cu,Zn superoxide dismutase as an example of a Greek key antiparallel β barrel. (a) α-Carbon stereo, viewed from one side of the barrel; (b) backbone schematic viewed as in a.
The commonest subgroup of antiparallel β barrel structures has a Greek key topology, with -3, +1, +1, -3 connections or a close variant. The first Greek key barrel structures were compared in Richardson et al. (), and they and the up-and-down barrels were described as categories in Richardson (). Figure 96 illustrates Cu,Zn superoxide dismutase as an example of a Greek key β barrel. There are 13 Greek key barrels in our sample, and 12 of them (all except staphylococcal nuclease) have the same handedness: viewed from the outside, the Greek key pattern forms a counterclockwise swirl (see Fig. 97). The four barrels shown in Figure 81 have a more complicated "jellyroll" topology with an extra swirl in the Greek key (this pattern was also common on Greek vases); the "jellyroll" Greek key topologies are shown in Fig. 98. The jellyroll pattern is produced by having a pair of connections, rather than just one connection, crossing each end of the barrel.
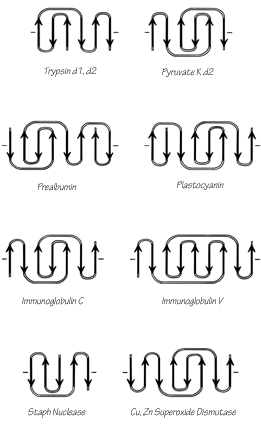
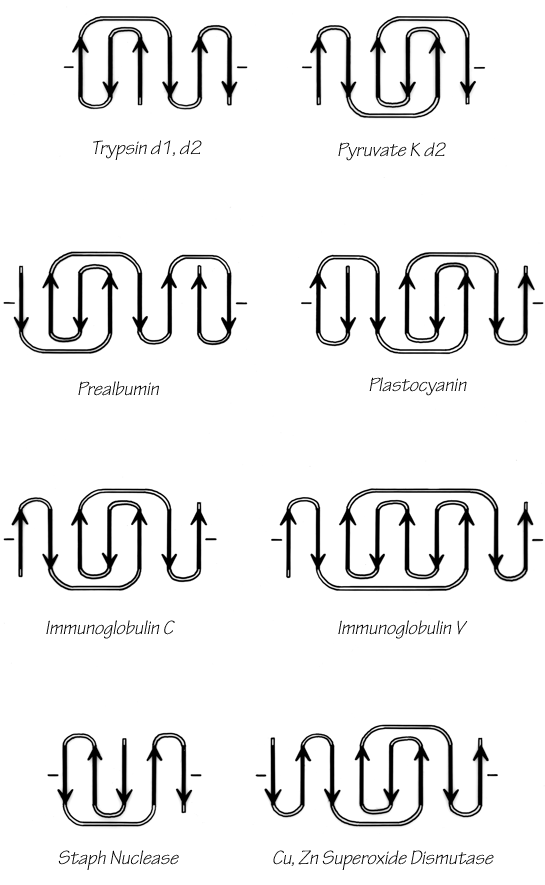
FIG. 97. Topology diagrams of the Greek key antiparallel β barrels. The dashes on either side of a topology diagram indicate that the barrel was opened up at that point and laid out Hat; all barrels are shown viewed from the outside.
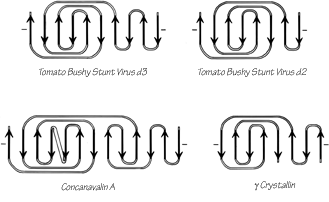
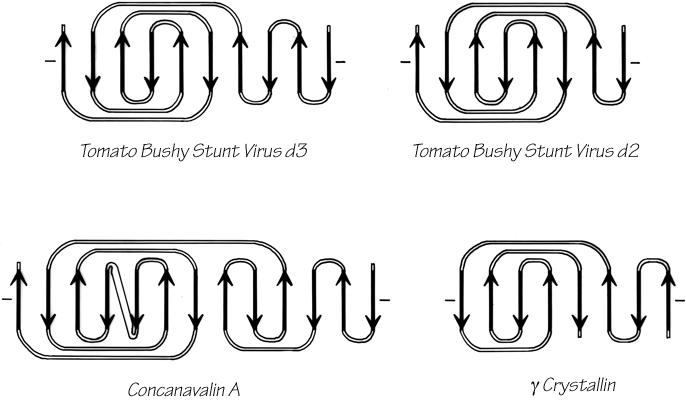
FIG. 98. Topology diagrams of the "jellyroll" Greek key β barrels.
The Greek key barrels have between 5 and 13 strands, but in all cases they enclose approximately the same cross-sectional area (see Section II,B). The cross sections are somewhat elliptical, with more flattening the more strands there are. For 8- to 10-stranded barrels, it is noticeable that the direction of the long axis of the cross-section twists from one end of the barrel to the other by close to 90° (see Fig. 99)
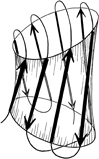
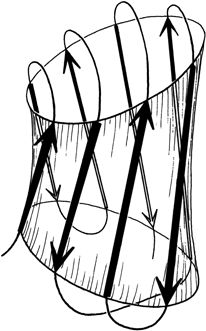
FIG. 99. A highly simplified sketch of the slightly flattened cylinder of a β barrel, showing how the direction of flattening twists from top to bottom.
± 3 connections are not particularly common outside of the barrels so that the prevalence of Greek key topologies is not due simply to chance combination of the connection types that make it up. There are two different ways of analyzing the Greek key which could perhaps explain both its frequent occurrence and its strongly preferred handedness. The first approach is to consider the stability of the final barrel, given its size, shape, and twist. Figure 99 shows that the Greek key pattern provides neat, efficient connections across the top and bottom of the barrel, lhydrogen bonding next to the ±1 connections. In tomato bushy stunt virus d3 there is actually some β-type hydrogen bonding between the +1, -3, and +5 connecting loops. In combination with the twist of the strands and of the barrel cross section, a counterclockwise Greek key (as shown) produces ±3 connections that are approximately perpendicular to each other on opposite ends of the barrel and that can both cross along a short axis of the cross section. A clockwise Greek key would place the ±3 connections in a weaker position approximately parallel to each other, and one of them would be along the long axis. This argument could not account for the handedness of the partial Greek keys with -3, +1, +1 topology (such as staphylococcal nuclease and chymotrypsin) where there is a ±3 connection at only one end of the barrel.
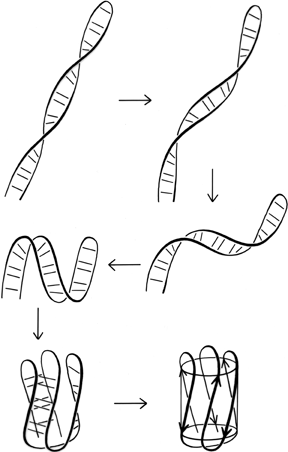
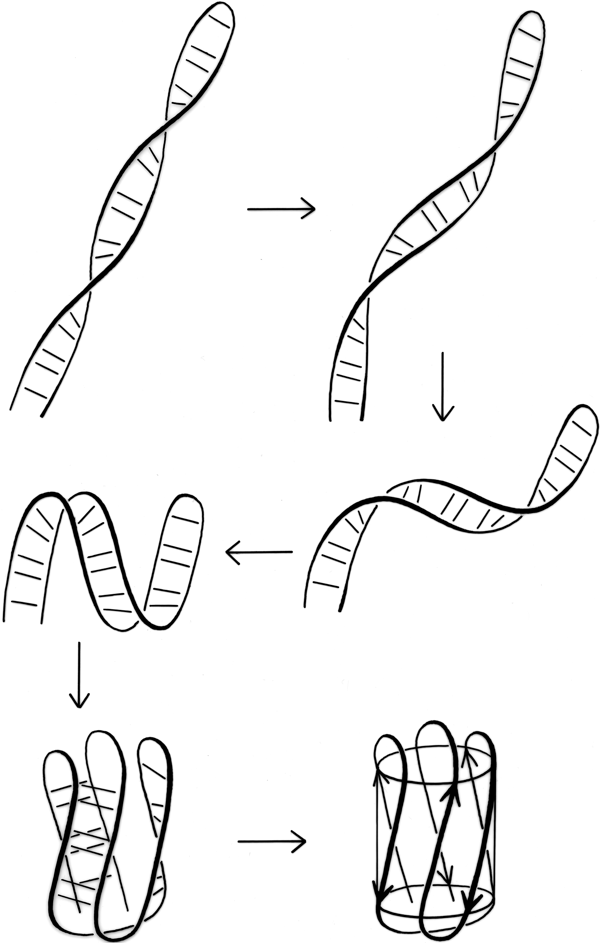
FIG. 100. A hypothetical folding scheme for Greek key β barrels which could explain why essentially all of the Greek key and jellyroll β barrels have the same handedness of topology.
The other possible explanation hypothesizes an effect during the protein folding process, very similar to the one proposed to explain crossover handedness (see Section II,B). All Greek keys, even the "jellyrolls," necessarily have a folding point halfway along the chain from which two paired strands can be followed back next to each other as they curl around the structure. Given the prevalence of Greek key patterns in the known structures, it seems very likely that the polypeptide chain can fold up by first folding in half and forming a long, two-stranded β ribbon, and then curling up the ribbon to produce the further β sheet interactions. This sort of process is illustrated in Fig. 100. Since the initial ribbon would presumably have a strong right-handed twist (see Section II,B), it would impart a right-handed twist to the curling direction and always end up with a counterclockwise Greek key. Besides the β barrels, there are other pieces of protein structure that suggest this sort of process, such as the long β ribbons in lactate dehydrogenase d2 (see Fig. 74). This kind of folding hypothesis has been utilized by Ptitsyn and Finkelstein () to obtain rather successful predictions of β strand contacts and topologies. [ Ray Salemme (1983) ref ?? explained the curling up of a long, 2-stranded ribbon by the fact that φ,ψ preferences are slightly different for β-hairpin residues between a narrow pair of H-bonds and those between a wide pair, such that the former prefer the concave side of a curl and tend to end up on the inside. ]
Partial, multiple, and other barrels have been grouped together as another subgroup within the antiparallel β category (see Fig. 82). Ribonuclease contains a four-stranded antiparallel β sheet that looks 1ike a five-stranded barrel with one strand missing. Alcohol dehydrogenase d1 includes a five-stranded antiparallel barrel (with a topology of +1, +3x, -2, +1) and another partial five-stranded barrel. Back-to-back β barrels that share one wall occur in the variable half of immunoglobulin Fab structures (except for Rhe: see ), where VL and VH are each antiparallel β barrels and the contact between them forms an even more regular eight-stranded barrel with four strands contributed from each domain (see Fig. 101). The three barrels pack against each other with a right-handed superhelical twist, and the angle between the axes of adjoining barrels is the same as the angle between opposite strands in one of the barrels. The two domains of the acid proteases have complicated, very similar mixed β sheets that could be described either as a six-stranded barrel with side sheets or as several interlocking β sheets. When more examples are available, it will probably be possible to find patterns to the ways in which small subsidiary β sheets can interlock into the edges of larger sheets (such as in the acid proteases or thermolysin d1), but for now no attempt has been made to classify them.
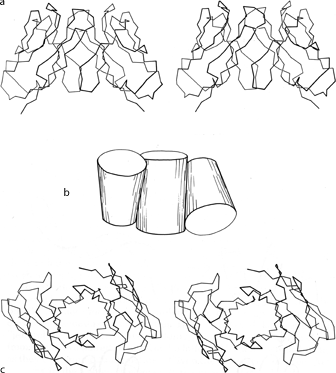
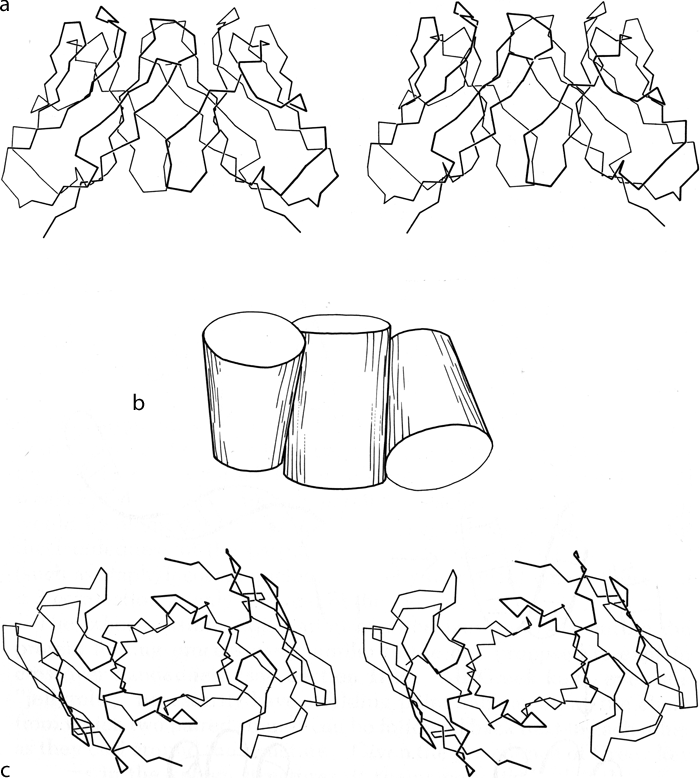
FIG. 101. Packing of two β barrel domains in the immunoglobulin VL. dimer (from Bence-Jones REI): (a) α-carbon stereo, viewed from the sides of the barrels; (b) simplified schematic of the barrels as cylinders, viewed as in a; (c) α-carbon stereo, viewed from one end of the barrels. The contact between the two domains forms a third barrel in the center.
The next subcategory of antiparallel β domains each has a single, more or less twisted β sheet, either pure antiparallel or predominantly so, but not closing around to form a barrel. They are shown in Fig. 83, and Fig. 102 shows glyceraldehyde-phosphate dehydrogenase as an example. Their common feature is a layer of helices and loops which covers only one side of the sheet, so that they are two-layer structures. Many β barrels have been described as "sandwiches," with two slices of β sheet "bread" and a "filling" of hydrophobic side chains; based on that analogy these structures would be "open-face sandwiches," with a single slice of β sheet "bread" and a "topping" of helices and loops. The open-face β sandwiches could rival a Danish buffet for variety on a theme: they range from 3 to 15 strands, with a wide assortment of topologies, curvatures, and placement of helices and loops. Bacterio-chlorophyll protein, the largest of them, encloses between the β sheet layer and the helical layer a core of seven bacteriochlorophyll molecules, tightly packed in an orderly but quite asymmetrical array.
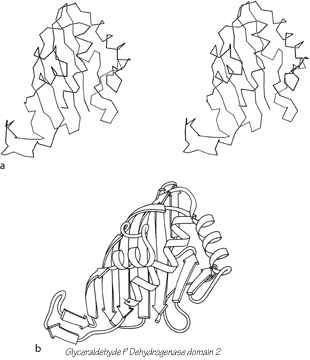
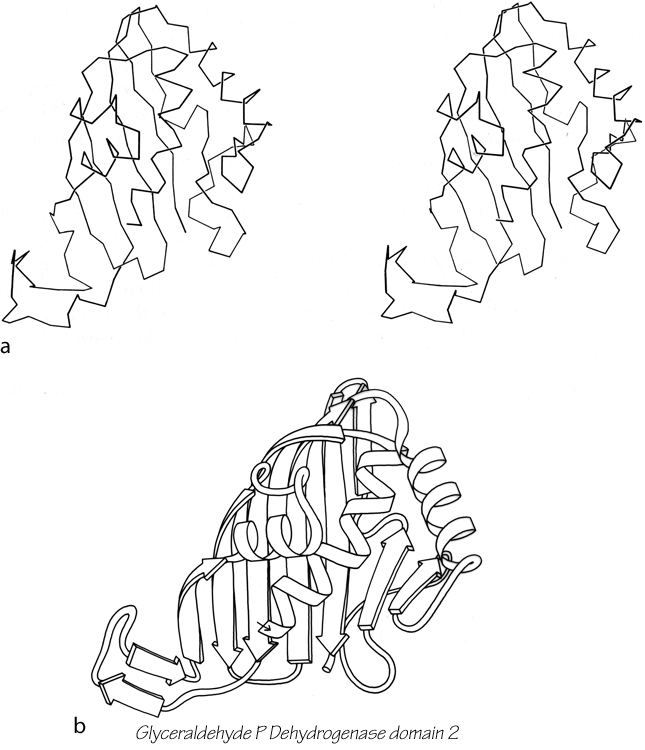
FIG. 102. Glyceraldehyde-phosphate dehydrogenase domain 2 as an example of an open-face sandwich antiparallel β sheet. (a) α-Carbon stereo, viewed from the buried side of the sheet; (b) backbone schematic, viewed as in a.
The remaining three antiparallel β structures form a miscellaneous category (see Fig. 84). Lactate dehydrogenase d2 and gene 5 protein each has several two-stranded antiparallel β ribbons, but they do not coalesce into any readily described overall pattern. The N-terminal domain of tomato bushy stunt virus protein has a unique β structure in which equivalent pieces of chain from three different subunits wrap around a 3-fold axis to form what has been called a "β annulus" (). Each of the three chains contributes a short strand segment to each of three three-stranded, interlocking β sheets. This "domain" provides one of the subunit contacts that hold the virus shell together. However, only one-third of the 180 subunits contribute to the β annuli; for the other quasi-equivalent subunits, the N-terminal part of the chain is disordered with respect to the virus shell.
[ Many new variants of all-β structures have been seen more recently, including two entirely unprecedented fold types. The β-propeller is made up of 4, 5, 6, 7, or 8 units, each a small 4-stranded up-and-down β sheet, arranged radially like propeller or rotor blades around a center of approximate symmetry (e.g., 1TBG G protein β subunit). The β-helix is a parallel all-β structure, which winds around in a shallow spiral forming 3 parallel β-sheets in a triangular cylinder. It comes in both righthanded (1O88 pectate lyase C) and lefthanded (1LXA Lpx A acyltransferase) forms, with different cross-sectional shapes, and is the only handed protein structure that commonly occurs in either handedness. ]